GMP Requirements - “The software development process should be sufficiently well planned, controlled, and documented to detect and correct unexpected results from software changes."
Validation is defined as the documented act of demonstrating that a procedure, process, and activity will consistently lead to the expected results. This is the formal testing to demonstrate that the software meets its specified requirements.
This is a documented process to verify that a computerized system does exactly what it is designed to do in a consistent and reproducible manner, that it will produce information or data that meets a set of defined requirements. If a system meets these requirements, it can be assumed that it is consistently performing in the way it was intended.
Validation helps to ensure that both new and existing computer systems consistently fulfill their intended purpose and produce accurate and reliable results that enable regulatory compliance, fulfillment of user requirements, and the ability to discern invalid and/or altered records.
Computer systems need to be examined to confirm that the systems will work in all situations.
Computer system validation is required when configuring a new system or making a change in a validated system (upgrades, patches, extensions, etc.).
Validation processes should be based on applicable regulations and guidance, best practices for the domain, and the characteristics of the system being validated.
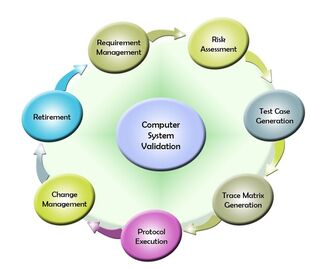
To validate software, it must be:
- structured, documented, and evaluated as it is developed;
- checked to make sure that it meets specifications;
- adequately tested with the assigned hardware systems;
- operated under varied conditions by the intended operators or persons of like training to assure that it will perform consistently and correctly.
Regulated industries have to adopt many compliance procedures to make sure their final product is safe for distribution or sale.
Regulatory agencies around the world require validation processes to confirm the accuracy and integrity of data in computerized systems in order to ensure product safety and effectiveness.
The process is used to make sure that the IT systems are completely transparent, robust and tamper proof because they directly impact public health and safety.
It is critical that these systems can be relied upon to produce data consistently and store those electronic data records so that they stand the test of time.
Validation is one of those compliance requirements and is part of the Quality Management System within regulated industries.
There are several of examples as to why software validation is important. We can look at our library of FDA Warning Letters to see more than 200 reasons to validate your software or systems.
There is a case study about Therac-25, a radiation therapy machine from the 1980s.
Due to programming issues, the machine could administer the wrong amount of radiation to patients (often as a huge overdose), which led to serious injuries and even death. Had there been software validation standards in place, these types of instances could have been identified and remediated prior to the treatment of patients.
Computer systems validation is serious and the FDA and other regulatory agencies do not take this lightly.
What benefits does validation deliver?
- Accuracy – when test outcomes are routinely checked against predetermined expected results, the accuracy of computer systems within the manufacturing process can be relied upon
- Security – validation processes make clear when entries to the system have been altered.
- Reliability – the process ensures that system outputs can be relied upon throughout the life cycle.
- Consistency – it also ensures that the system output is consistent across its life cycle.
- Optimization – following the process also means that computer systems can be more easily optimized. Optimization is a key feature of an effective and efficient manufacturing site.
 RSS Feed
RSS Feed
